The innate immune system is the first line of defense against threats and relies on pattern recognition receptors (PRRs) to recognize conserved molecular structures of pathogens. Macrophages play a key role in this inflammatory response by activating the NLRP3 inflammasome. The NLRP3 inflammasome complex consists of NLRP3, ASC, and pro-caspase-1, and its activation leads to the production of pro-inflammatory cytokines. More specifically, ASC, a protein involved in cell death and inflammation, is involved in the conversion and maturation of key pro-inflammatory cytokines and caspase-1. ASC is also involved in the development of certain tumors. However, the expression of the inflammasome complex in response to drugs used in cancer treatment remains to be investigated. In this study, a droplet-based microfluidic platform was used to examine the link between apoptosis (cell death) and inflammasome processing in response to treatment with temsirolimus (mTOR inhibitor), rifabutin (transcription inhibitor), and BAY 11-7082 (NFκB inhibitor). THP-1 cells with fluorescently tagged ASC were exposed to these cancer drugs, and the heterogeneous response of individual cells was analyzed. The authors found that THP-1 cells increased expression of ASC in response to treatment with temsirolimus, rifabutin, and BAY 11-7082 in >60% of cells, suggesting that the apoptotic pathway was activated in these cells. These findings demonstrate that cancer drugs only activate cell death in a portion of cells, providing valuable insights into cell-to-cell variability and potential applications for personalized medicine.

In order to establish baseline ASC expression, the authors first cultured THP-1 cells in droplets in the absence of any drugs or inhibitors. Over 24 hours, the cell count did not change. However, ASC expression as measured by GFP intensity decreased over time. This suggested that without any stimuli, THP-1 cells did not activate the NLRP3 inflammasome as seen by decreased ASC expression. Single-cell analysis revealed that there was significant cell-to-cell heterogeneity. ASC expression was categorized into increasing, constant, or decreasing trends. Over 50% of THP-1 cells exhibited a decreasing trend in ASC expression. In contrast, 7.1% of the cells showed an increasing trend and 35.7% had a constant trend in ASC expression. These findings also demonstrate that the majority of THP-1 cells remained vital because high expression of ASC indicates that cells may be undergoing apoptosis.
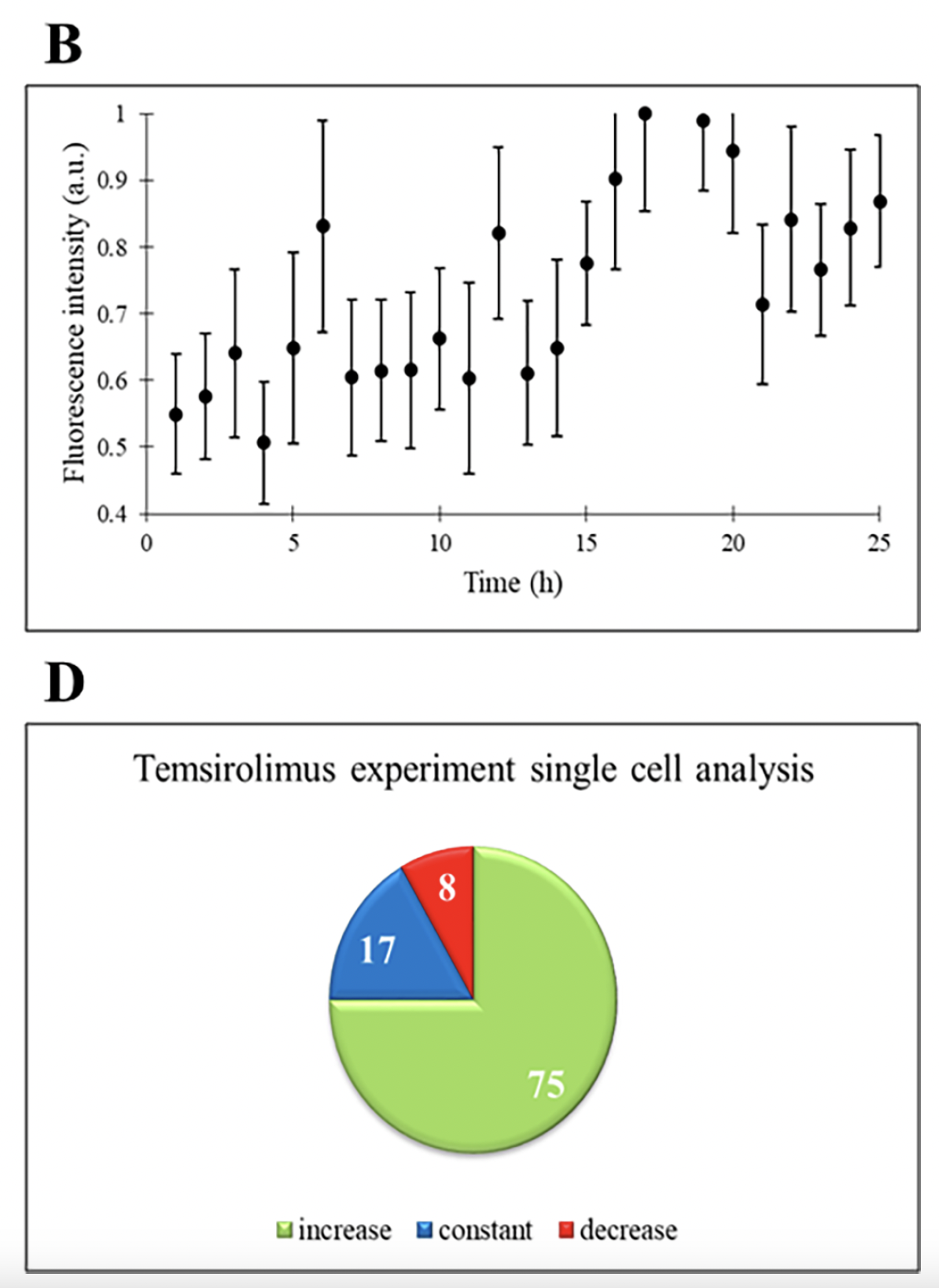
Next, the authors challenged THP-1 cells with temsirolimus, an FDA-approved drug used for the treatment of various malignancies and solid tumors. Temsirolimus inhibits the mTOR pathway, which plays a crucial role in cell growth, proliferation, and survival. Dysregulation of mTOR is implicated in the development of several cancer types. When THP-1 cells were treated with temsirolimus, there was no change in cell count, similar to the control experiment. However, there was an increasing trend in the normalized fluorescence intensity, indicating increased expression of ASC. This suggests that temsirolimus induces the apoptotic pathway in THP-1 cells, as ASC is involved in cell death processes. Individual cell analysis showed that the majority of cells exhibited increased ASC expression (75%), again indicating the drug's effectiveness in promoting apoptosis. The remaining 17% were constant and 8% had decreasing trend in ASC expression, suggesting that these cells were not responsive to temsirolimus treatment.
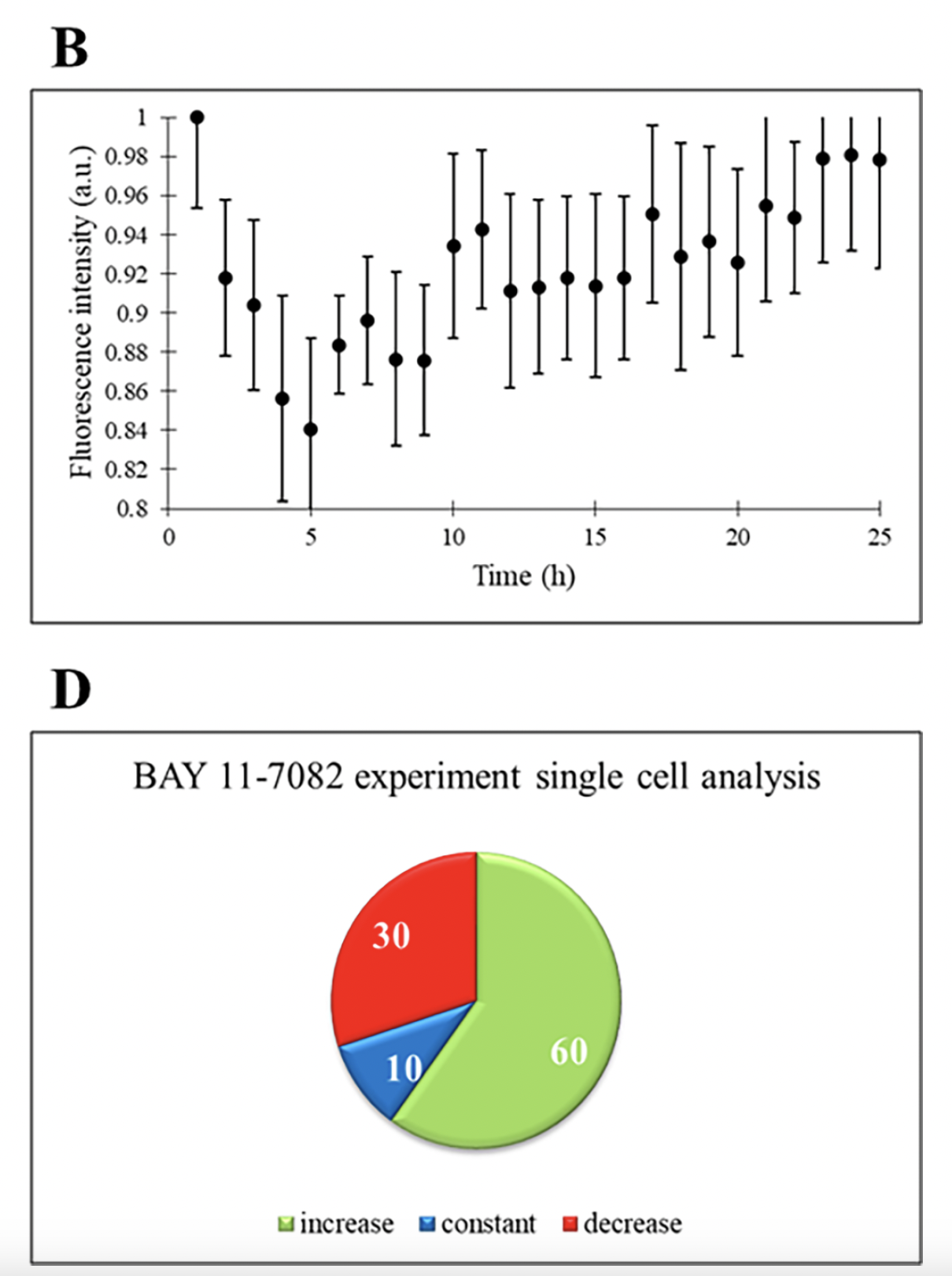
The transcription factor NFκB regulates the expression of numerous genes involved in immune regulation, cell survival, differentiation, and proliferation. Dysregulated NFκB activity is associated with diseases including tumor development and metastasis. Therefore, anti-cancer drugs have targeted NFκB inhibition. BAY 11-7082 is an anti-inflammatory drug that acts as an inhibitor of NFκB expression. When BAY 11-7082 was administered to THP-1 cells, there was no change in cell count. Similar to the temsirolimus experiment, an increase in ASC expression was observed on a population level. However, single-cell analysis shows that only 60% of the cells exhibited an increased expression of ASC throughout the experiment, indicating apoptosis induction. The remaining 30% showed decreasing expression and 10% had constant expression of ASC. In a separate experiment where THP-1 cells were treated with rifabutin, an RNA synthesis inhibitor, increased expression of ASC was only detected in 50% of cells. Together, the use of the droplet microfluidic platform in this study revealed that the heterogeneous expression of ASC occurs at the single-cell level, suggesting that a significant portion of THP-1 cells did not respond to the anti-cancer drug. THP-1 cell line was used in this biochemical study for their similarities to human monocytes and ease with passaging. However, further investigation is needed to examine this cellular heterogeneous response to anti-cancer drugs in primary cancer cells.


