Protein nanoparticles are ideal candidates for use as drug delivery systems due to their unique size and biocompatibility. However, current methods of generating nanoparticles may be high throughput but have poor control over size and uniformity. To address these limitations, Zhang and colleagues used a droplet-microfluidic approach to generate protein-based nanoparticles within droplets. This method enabled the production of homogenous protein-based nanoparticles suitable for intracellular delivery. By controlling parameters such as flow rates and protein concentration, the size and distribution of the nanoparticles could be regulated. Nanoparticles made of reconstituted silk fibroin, bovine serum albumin, and beta-lactoglobulin were highly compatible for cellular uptake with minimal toxicity in human embryonic kidney cells. These protein-based nanoparticles were stable even after 60 days. These findings support the use of protein-based nanoparticles as drug delivery systems due to their high biocompatibility and efficient cellular uptake. In summary, the study demonstrated the feasibility of using droplet microfluidics to produce protein-based nanoparticles with desirable properties for biomedical applications.
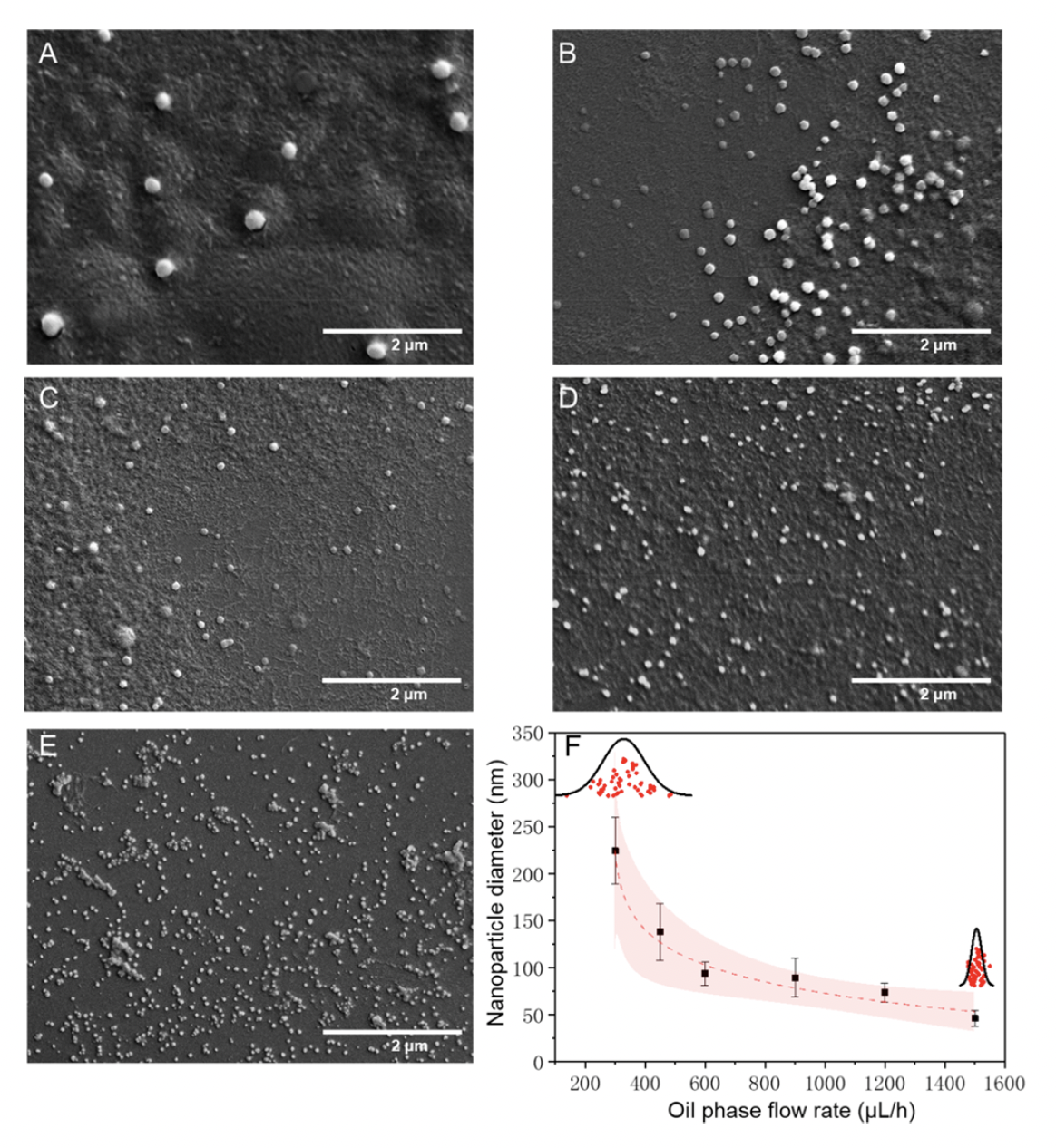
Zhang et al. utilized a droplet microfluidic platform consisting of oil as the external phase, ethanol as the middle phase, and protein as the internal phase. Ethanol acted as a desolvation agent, leading to protein nucleation and nanoparticle formation. Mixing within the droplets facilitated formation of uniform protein-based nanoparticles. Proteins tested included reconstituted silk fibroin, bovine serum albumin, and beta-lactoglobulin. The authors next optimized synthesis by investigating the effect of protein concentration, oil phase flow rate, and ratio of the two aqueous phases on nanoparticle size and uniformity. Protein concentration less than 0.5 mg/mL resulted in 80 nm particles. However, beyond the critical concentration of 0.5 mg/mL, the particle size increased from 100 nm to 350 nm. Increasing the ethanol-to-protein flow rate ratio decreased particle size from 250 nm to 160 nm, improving uniformity. A ratio greater than 2 resulted in an increase in particle size (160 nm to 220 nm) without significant impact on particle distribution. This suggested that nanoparticle size varied based on the ethanol-to-protein ratio. Finally, as the oil phase flow rate increased, the nanoparticle size decreased exponentially while uniformity increased as seen with scanning electron microscopy (SEM) results. Overall, droplet microfluidics enabled Zhang and colleagues to generate nanoparticles in a high-throughput manner with optimal uniformity.
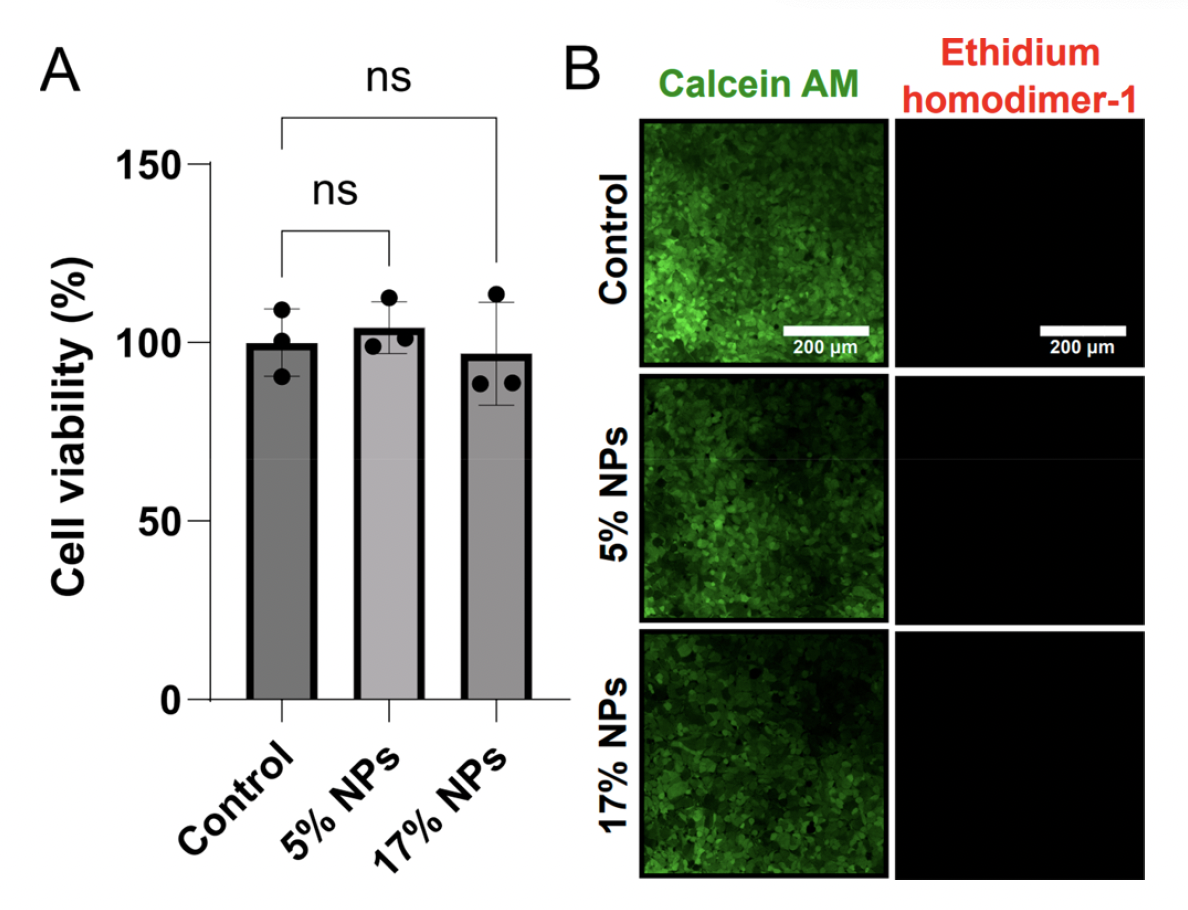
To establish that protein-based nanoparticles were biocompatible, Zhang et al. next examined cytotoxicity and cell viability of human embryonic kidney cells (HEK-293) when exposed to protein-based nanoparticles. HEK-293 cells were cultured overnight with ~29 nm wide silk-based nanoparticles at 5% and 17% concentrations. Cell viability was determined with an MTT-based viability assay. Results demonstrated that cell viability in cells treated with 5% or 17% nanoparticles was similar to the untreated controls. Furthermore, the authors used calcein AM vs ethidium homodimer-2 staining to differentiate live vs dead cells, respectively. From fluorescent imaging, there was no significant differences in cell viability when HEK-293 were treated with silk-based nanoparticles. The authors also determined that the size of the nanoparticles and protein also did not affect cell viability. These findings suggest that protein-based nanoparticles demonstrate high biocompatibility with human cells and could be used as drug delivery systems.
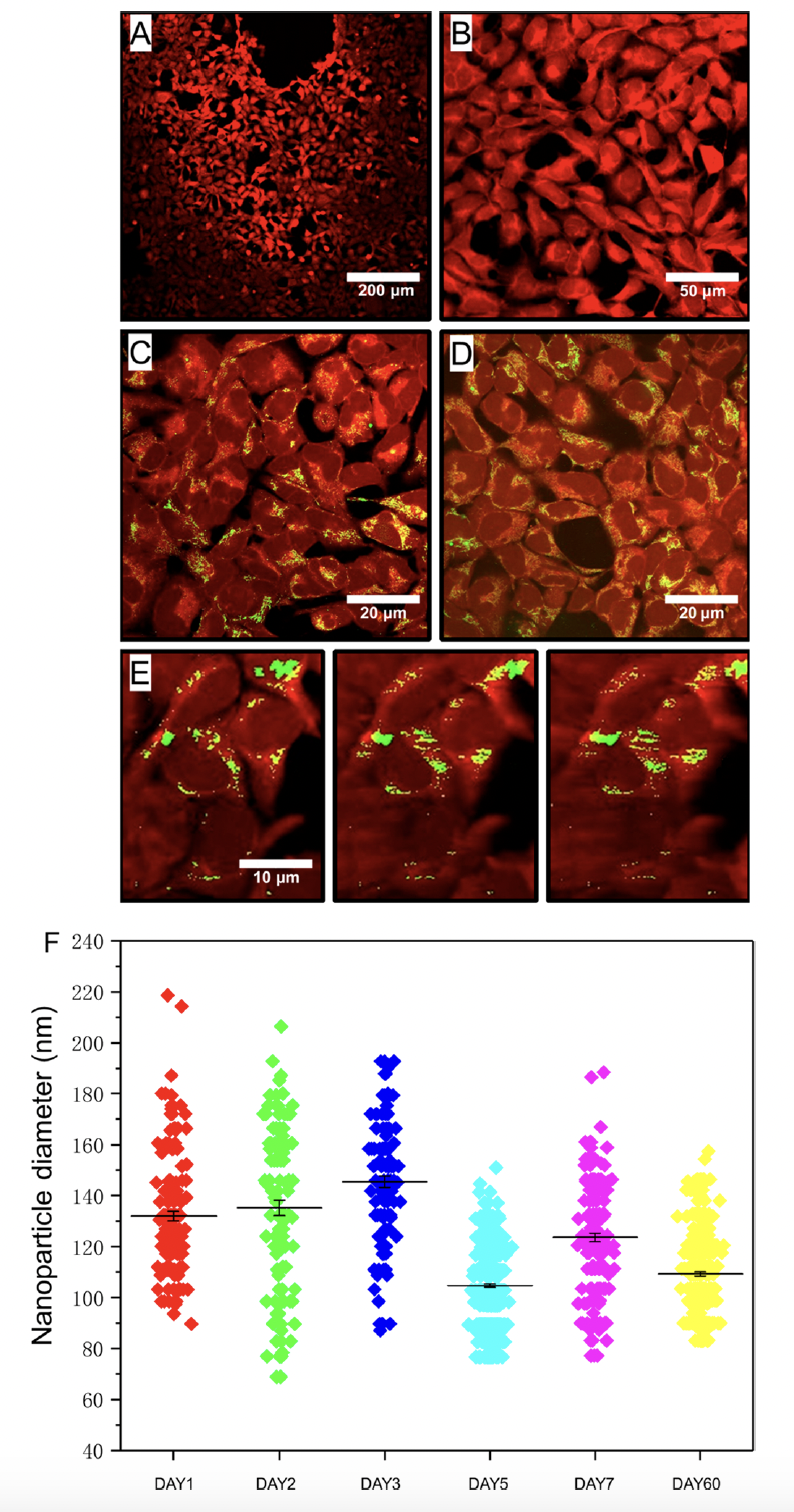
To demonstrate that protein-based nanoparticles could be readily absorbed by cells, the authors next used confocal microscopy to track nanoparticle localization within HEK-293 cells. Cells were stained with CellTracker Violet BMQC due while nanoparticles were tagged with Atto 488 (green). HEK-293 cells and nanoparticles were cocultured as previously done. As expected, cell controls without nanoparticles did not exhibit any fluorescence signal at the characteristic peak wavelength of 520 nm for the dye-tagged nanoparticles. However, in the presence of nanoparticles, green areas were observed, clearly overlapping with the cells. Cellular uptake of protein-based nanoparticles was observed for both nanoparticle concentrations (5% and 17%). Confocal images showed that almost all cells contained nanoparticles, indicating close to 100% intracellular uptake. The authors also tracked uptake longitudinally. SEM micrographs were taken after 1, 2, 3, 5, 7, and 60 days, and the results showed that the nanoparticles remained stable with consistent sizes and uniformity over the entire duration. The particle morphology remained smooth and spherical throughout the stability testing. These findings indicate that the nanoparticles have excellent stability, making them suitable for long-term applications. In summary, the authors leveraged droplet microfluidics to produce uniformed protein-based nanoparticles that were readily absorbed by human cells with low cytotoxicity. These proteins demonstrate that protein-based nanoparticles are highly suitable for drug delivery applications.


